Study provides important insights for future predictions of SARS CoV-2 infection
Published: 2022-02-21
The outbreak of SARS CoV-2 has increased the demand for developing large, population-based immunological surveys. These are needed to assess long-term immune responses following COVID-19 infection. Previous studies have shown that the majority (91–99%) of COVID-19 cases produce an antibody response (seroconvert). After recovery from a COVID-19 infection, relatively stable levels of neutralizing IgG antibodies are detected in blood for up to eight months. In addition, SARS-CoV-2-specific T-cell responses are detected, with a shorter duration of CD8+ T-cell responses and a longer duration of CD4+ T-cell responses.
Various serological assays were rapidly developed and implemented early in the pandemic. Today, these are available both through the healthcare system and, for example, in pharmacies. However, development of cellular assays measuring T-cells response is more difficult and time-consuming. One limiting factor towards the development of cellular assays measuring T-cells as a means of detecting prior infection with SARS-CoV-2 has been the risk of cross-reactivity due to prior encounters with human coronaviruses (HCoVs). This cross-reactivity could potentially affect assays specificity.
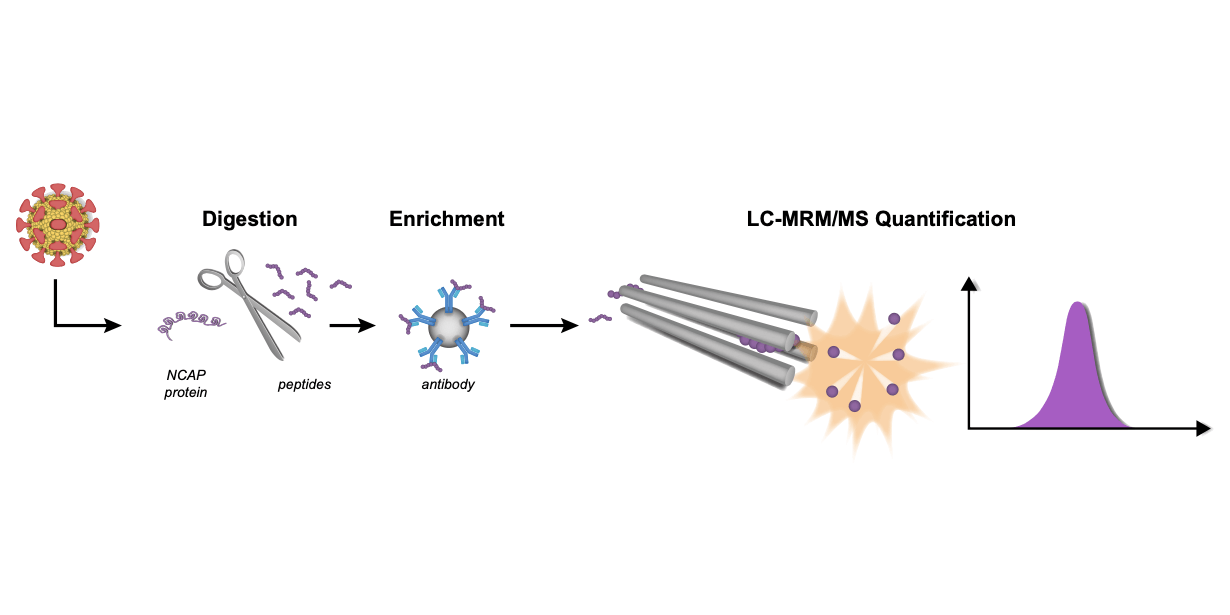
In a recently published study by Sara Mangsbo (first author: Sara Mangsbo, Uppsala University/Science for Life Laboratory; corresponding author: Charlotte Thålin, Karolinska Instituet/Danderyd Hospital) characterized the cellular responses to a set of SARS-CoV-2 peptide pools in order to validate a SARS-CoV-2 IFN-γ Release Assay (IGRA) based on the FluoroSpot method.
Mangsbo and colleagues used both commercially available SARS-CoV-2-specific peptide pools, and an “in-house” designed SARS-CoV-2 peptide pool. The in-house peptide pool was restricted to five amino acid aligning with endemic HCoVs. The researchers obtained blood samples from the ongoing COMMUNITY study (COVID-19 Biomarker and Immunity, funded among others by the SciLifeLab/KAW National COVID-19 Research Program), which investigates long-term immunity after COVID-19 infection and vaccination in healthcare workers. The results showed that the in-house generated peptide pool had a relatively high specificity of 96.1% compared to the two commercially available peptide pools with specificities of 80.4% and 85.3%, respectively. The commercially available peptide pools showed sensitivities of 92.0% and 66.8%, respectively, compared to a specificity of 76.0% for the in-house generated peptide pool.
In summary, the sensitivity and specificity of a FluoroSpot SARS-CoV-2 T-cell assay was evaluated using three different SARS-CoV-2 peptide pools. The study found the T-cell assay to have limited clinical use due to low sensitivity. However, the researchers highlight that future diagnostic tools build on T cell receptor sequencing, to identify T-cells that recognize SARS-CoV-2 antigens, may be useful for prediction of previous infections. In the US one such test has already been given an emergency use authorization by the U.S. Food and Drug Administration.
SciLifeLab/KAW National COVID-19 Research Program
The aim of the SciLifeLab/KAW National COVID-19 research program is to provide useful knowledge about the current situation during the corona pandemic and also to provide knowledge for future pandemics. In addition, the program aims to improve preventions, diagnostics and treatments of viral disease, a clearer real-time overview of the pandemic, integrate data across technologies, and research domains, and create better predictive models. This research program is made possible by funding from Knut and Alice Wallenberg Foundation (KAW). More about the COVID-19 efforts funded by KAW here.Data
- Information about the flourospot peptide sequences used is available under DOI: 10.1371/journal.pone.0258041.s001 and DOI: 10.1371/journal.pone.0258041.s002.
- Because sensitive data cannot be openly shared, the COMMUNITY study dataset has a metadata record set up in the SciLifeLab Data Repository, DOI: 10.17044/scilifelab.13567355.v2.
Article
DOI: 10.1371/journal.pone.0258041
Mangsbo SM, Havervall S, Laurén I, Lindsay R, Jernbom Falk A, et al. (2021) An evaluation of a FluoroSpot assay as a diagnostic tool to determine SARS-CoV-2 specific T cell responses. PLOS ONE 16(9): e0258041
Funding
The project that gave rise to these results received from the Swedish Research Council, SciLifeLab/KAW National COVID-19 Research Program, The Heart and Lung foundation, Leif Lundblad Family Foundation, Region Stockholm, Erling Persson Family Foundation, and Svenska Sällskapet för Medicinsk Forskning